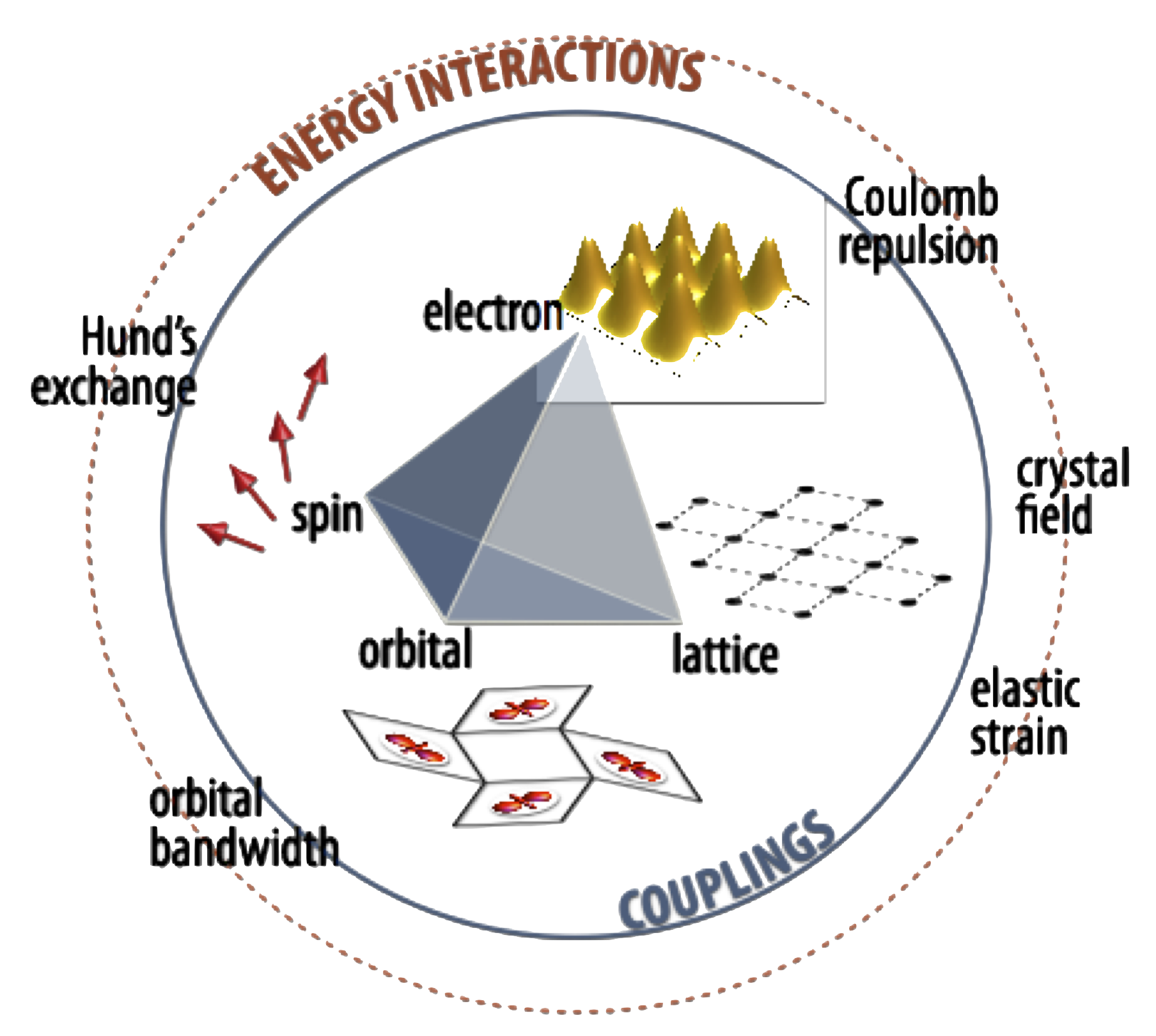
 Fig. from J.M. Rondinelli and N.A. Spaldin, Advanced Materials 23, 3363-3381 (2011).
Fig. from J.M. Rondinelli and N.A. Spaldin, Advanced Materials 23, 3363-3381 (2011).
Complex oxides are solid-state materials that contain oxygen and other elements, typically including a transition metal, then referred to as transition metal oxides. This class of materials host a wide-range of exciting properties including superconductivity, colossal magnetoresistance, ferromagnetism, ferroelectricity, thermal metal-insuator Mott transitions, and many others. The electrons within these materials defy traditional materials understanding in terms of band theory due to the very strong correlation effects, leading to coupling of the charge, spin, orbital, and lattice degrees of freedom.
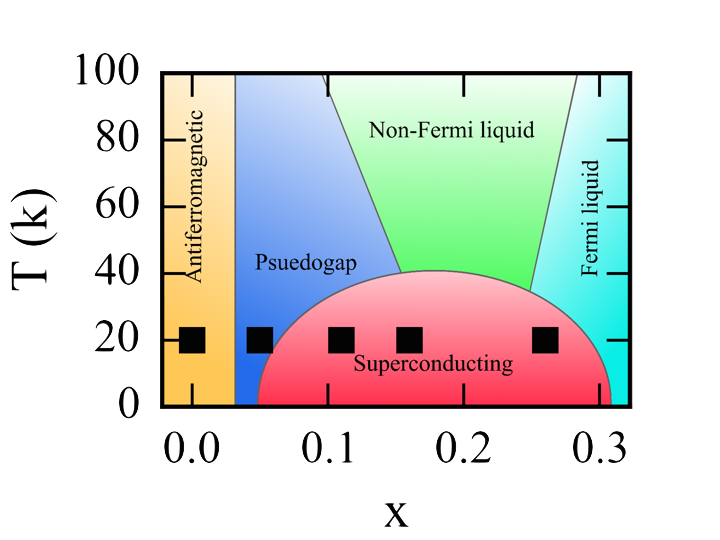 Cuprate phase diagram for La2-xSrxCuO4
Cuprate phase diagram for La2-xSrxCuO4
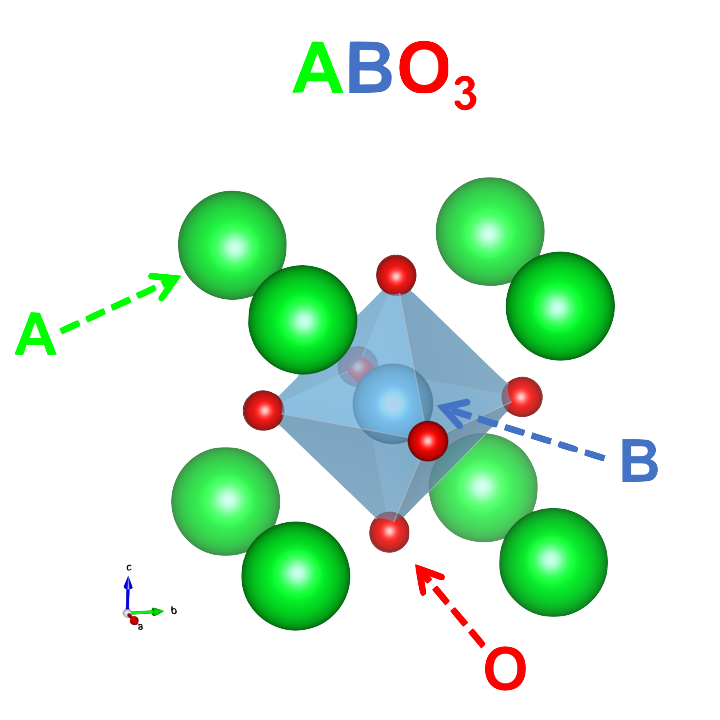
The typical formula for these materials is ABO3. The A-site is usually a lanthanide element which serves mostly as a space filling ion, B is typically a transition metal where most of the interesting physics occurs, and O is the anion which varies in importance to the observed properties. These materials are exceptionally chemically flexible, with exceedingly numerous A- and B-site combinations available. Doping of either the A-site or B-site, or both, is routinely achieved and leads to exceptionally complex phase diagrams highlighting the senstive interplay of the different microscopic interactions of these systems. The cuprates are the paragon of this area, with a complex phase diagram including highly varied and interconnected states, including the infamous high-temperature superconducting phase. Owing to the highly complex nature of the interactions in these systems, 30 years of rigorous study has failed to decisively identify the mechanism that generates the superconducitng charge carriers, known as Cooper pairs. During this same period, several other anomalous superconducting materials have been found further complicating the attempts to understand this state which could revolutionize electronics and power consumption.
Interfaces of complex oxides
As stated above, bulk complex oxides already host intrguing properties that still defy complete explanation and are a very actice area of research today. However, the realization of synthesis methods with atomic layer precision has revealed even more exciting behavior that derives from the interfaces between different oxide materials, so-called emergent properties. The most famous example of this phenomena is the interface of LaAlO3 and SrTiO3. Both of these materials are fairly mundane band gap insulators, however when an interface between the two is created a two-dimensional electron gas superconducting state is realized. Several other interesting behaviors at oxide interfaces have been found as well. More recently, interfaces of complex oxides and non-oxide materials have also lead to extremely exiciting observations. The most prominent of these findings replaces the LaAlO3, mentioned above, with the iron-based superconductor FeSe. Interfacing FeSe and SrTiO3 in this way raises the superconducting transition temperature in the FeSe layer from around 8K, to around 80K, an order of magnitude increase! This has helped establish interfacial oxides as some of the most intriguing and challenging materials available for study today.
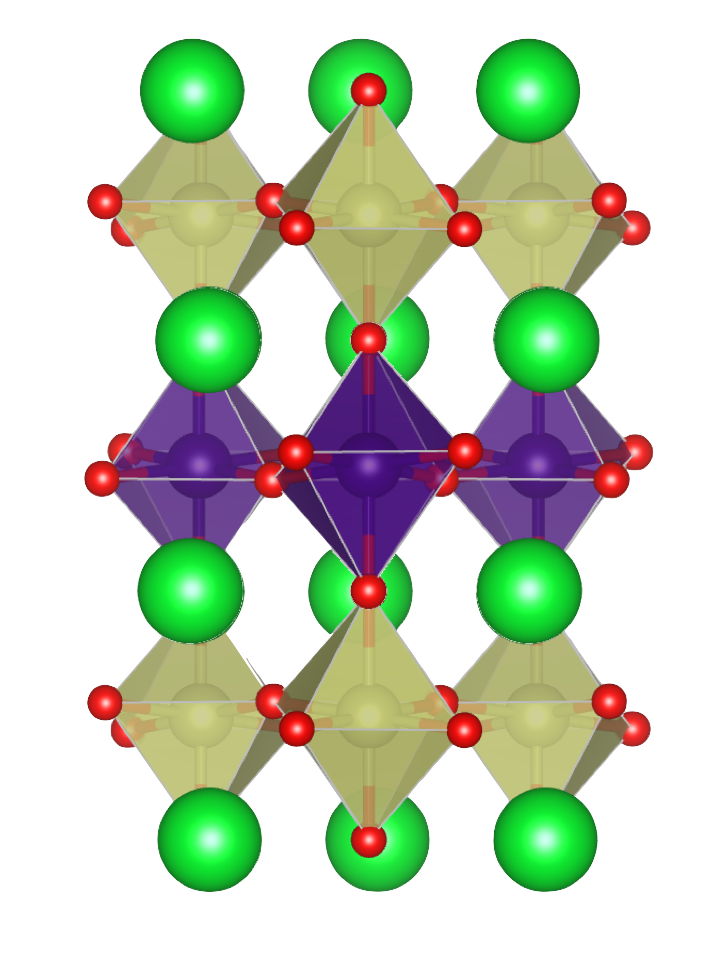 Example of a superlattice structure with SrTiO3 layers (top and bottom) sandwiching a SrIrO3 layer (center), creating an interfacial material.
Example of a superlattice structure with SrTiO3 layers (top and bottom) sandwiching a SrIrO3 layer (center), creating an interfacial material.
